Comparison of residual carbon content and morphology of B4C powders synthesized under different conditions
- 1 Ceramics Department, Materials and Energy Research Center (MERC), Karaj, Iran
Abstract
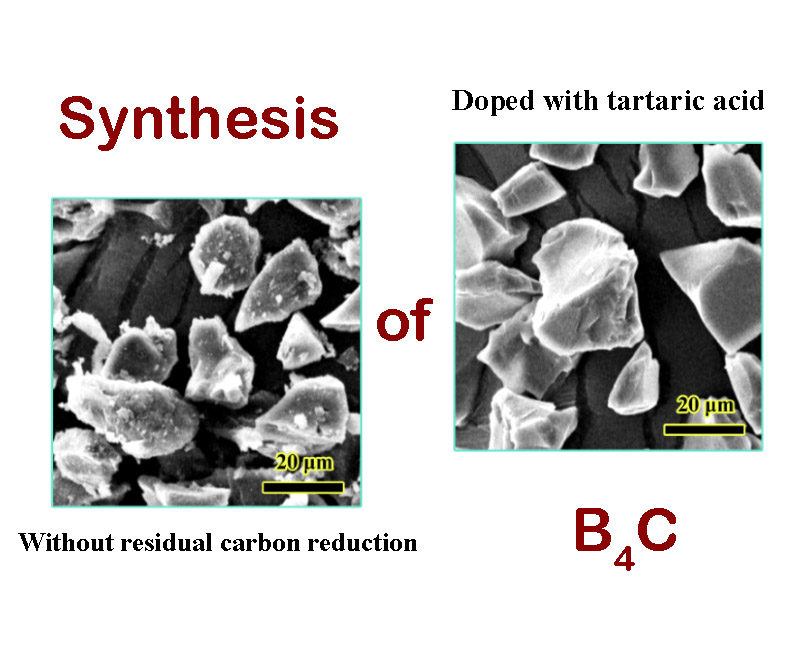
In this article, the impact of different B4C synthesis methods on the amount of residual carbon and the final morphology of the prepared ceramic particles was investigated. The main materials for the synthesis of B4C were glucose and boric acid, and the effects of adding tartaric acid and performing mechanical activation were studied. For this purpose, two methods of carbon dissolution and boron carbide oxidation were used to determine the amount of residual carbon in the ceramic products. The results of the investigations on the sample synthesized in optimal conditions showed that if additives and mechanical activation are not used, about 7 wt% of carbon will remain in the synthesized powder. The amount of carbon decreased to 5.7 wt% with mechanical activation, but the best result was obtained with the addition of tartaric acid, in which the amount of impurity dropped to 3.3 wt%. Finally, the size and morphology of B4C particles and carbon impurities were observed and compared using a scanning electron microscope.
Downloads
References
Copyright (c) 2023 Seyed Faridaddin Feiz, Leila Nikzad, Hudsa Majidian, Esmaeil Salahi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












