Non-catalytic applications of g-C3N4: A brief review
- 1 Department of Mechanical Engineering, University of Mohaghegh Ardabili, P.O. Box 179, Ardabil, Iran
Abstract
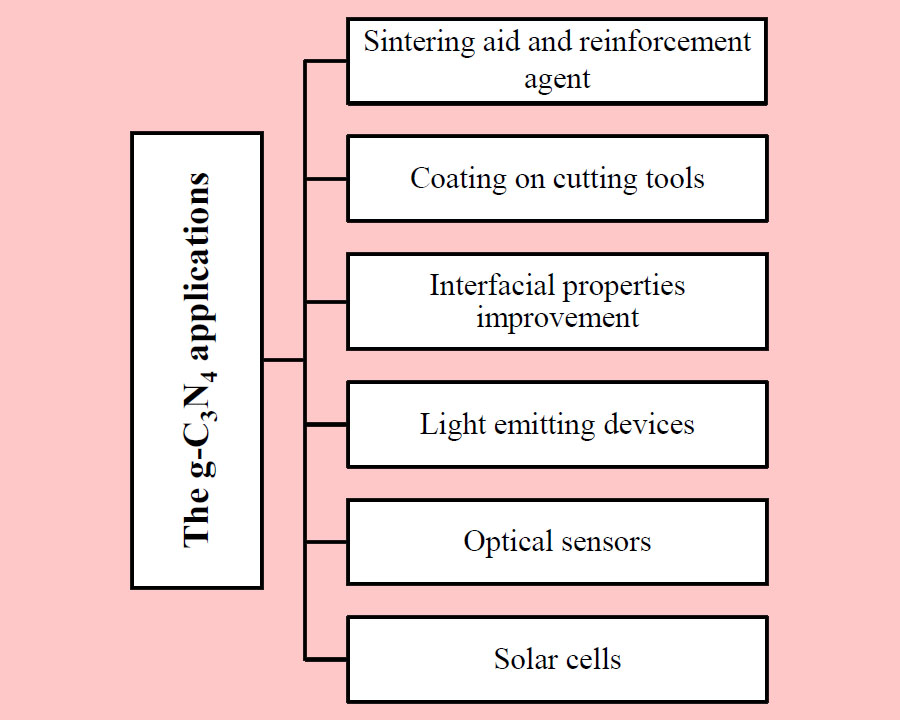
The g-C3N4 which is well known as a polymeric non-metal semiconductor, has been fabricated by thermal polymerization. It has also been used in catalytic applications including, photo-catalysis, removal and degradation of pollutants in water, Friedel-Crafts reactions, oxygen reduction reaction and etc. It has drawn noticeable research attention due to its economical and affordable fabrication, non-toxicity, biocompatibility, good thermal and electrical conductivity, high hardness, Corrosion resistance, and fireproofing properties. Therefore, the g-C3N4 has found non-catalytic applications including composites, cutting tools, improving surface properties, light emitting devices, optical sensors, and solar cells. In the current review, the novel and non-catalytic applications of g-C3N4 have been highlighted.
Downloads
References
Copyright (c) 2022 Milad Sakkaki, Seyed Mohammad Arab

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












