Evolution of red ceramic pigments: from hazardous compounds to environmentally friendly alternatives
- 1 Department of Materials Engineering, Naghshejahan Institute of Higher Education, Baharestan, Isfahan, Iran
- 2 School of Materials Science and Engineering, Jingdezhen Ceramic University; Jingdezhen 333403, China
- 3 Department of Inorganic Pigments and Glazes, Institute for Color Science and Technology (ICST), Iran Ministry of Science, Research and Technology, PO Box: 1668814811, Tehran, Iran
- 4 Sao Carlos Institute of Physics, University of Sao Paulo - USP, Sao Carlos - SP, Brazil
- 5 School of Materials Science and Engineering, Shaanxi University of Technology, Hanzhong, Shaanxi, Postal code: 723001, Shaanxi, China
- 6 The Wroblewski Library of the Lithuanian Academy of Sciences, Document Conservation and Restoration Department, Žygimantų str. 1, Vilnius, LT 01102, Lithuania
- 7 Ceramic Technology Department, Escola Superior de Ceràmica de L’Alcora (ESCAL-ISEACV), 12110 L’Alcora, Spain
Abstract
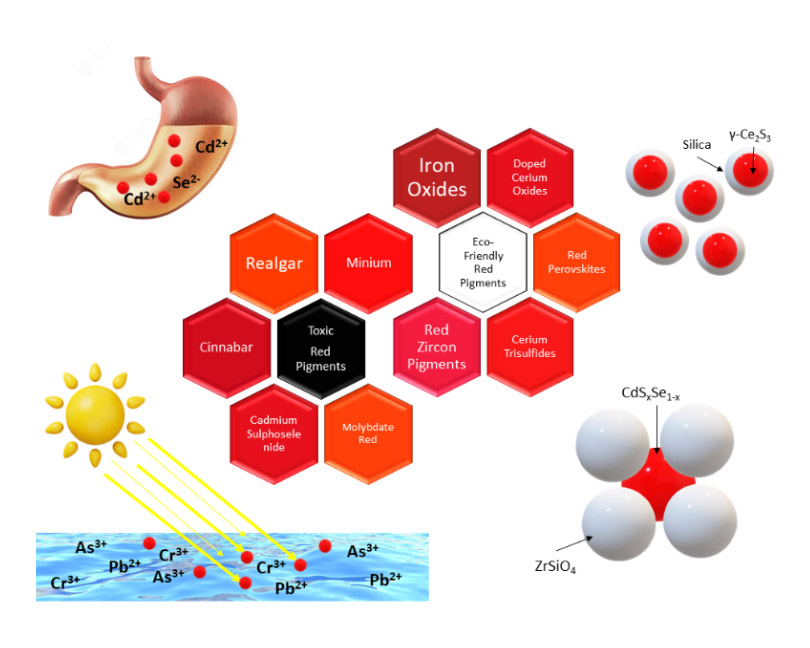
Synthesis of red ceramic pigments is a challenging task in the ceramic industry. Most classic reds are based on severely toxic materials including lead, arsenic, mercury, selenium, and cadmium, which are forbidden in many countries. On the other hand, the red color is super sensitive to the synthesis parameters, heat treatment conditions (atmosphere and temperature), particle size, etc. Therefore, achieving a bright true red shade and its stability at high temperatures is crucial. There has been a massive attempt to find a sustainable high-temperature resistant alternative for these hazardous compounds. Iron oxide is one of the first red pigments in history, but it cannot produce a bright red shade and its color is mostly red-brown. Ce2S3 is another red pigment with a beautiful red color. But it cannot stand the temperature above 350 °C in an oxidizing atmosphere. Doping lanthanides in the perovskites or entrapping the toxic beautiful chromophores in the core-shell structures are among the strategies to achieve safe bright red pigments. This review outlines the recent progress of hazardous classic reds to environmentally friendly ceramic red pigments. Various compounds and dopants, applied to develop sustainable reds, from simple iron-oxides to composites, solid solutions, core-shell structures, or even purified wastes have been covered in this review.
Downloads
References
Copyright (c) 2024 Rayehe Tavakolipour, Yueming Li, Maryam Hosseini Zori, Maria Ines Basso Bernardi, Kun Li, Aušra Čiuladienė, Eva Miguel

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












