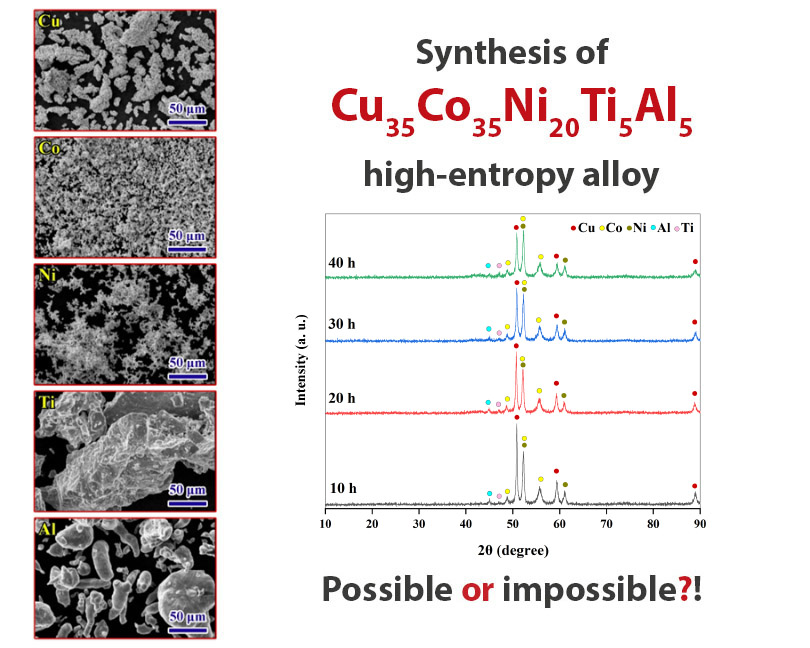
Is synthesizing a Cu35Co35Ni20Ti5Al5 high-entropy alloy beyond the rules of solid-solution formation?
- 1 Department of New Science and Technology, Nanomaterials Group, Semnan University, Semnan, Iran
- 2 Department of Materials and Metallurgical Engineering, Semnan University, Semnan, Iran
Abstract
In this research, an attempt was made to produce multi-component nanocrystalline Cu35Co35Ni20Ti5Al5 alloy by mechanical alloying. To produce this high-entropy alloy, the primary powders were milled for 40 h and characterized by XRD, SEM, EDS, and DSC analyses. The milling process has reduced the size of the crystallites to the nanometer scale and a nanostructured multicomponent powder with a crystallite size of 29 nm was obtained. According to the XRD patterns and EDS maps of the milled powder for the longest time, aluminum and copper were homogeneously distributed, cobalt had a less homogeneous distribution than these two elements, but nickel and titanium remained in concentrated spots. Finally, thermodynamic calculations were done to clarify the reason for the impossibility of forming a solid solution for the synthesis of the Cu35Co35Ni20Ti5Al5 high-entropy alloy.
Downloads
References
Copyright (c) 2023 Samaneh Mamnooni, Ehsan Borhani, Hassan Heydari

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.