Impact of bridging oxygens formation on optical properties of Fe3+ doped Li2O–Al2O3–SiO2–TiO2 glasses
- 1 Ceramic Department, Materials and Energy Research Center (MERC), P.O. Box 31779-83634, Karaj, Iran
Abstract
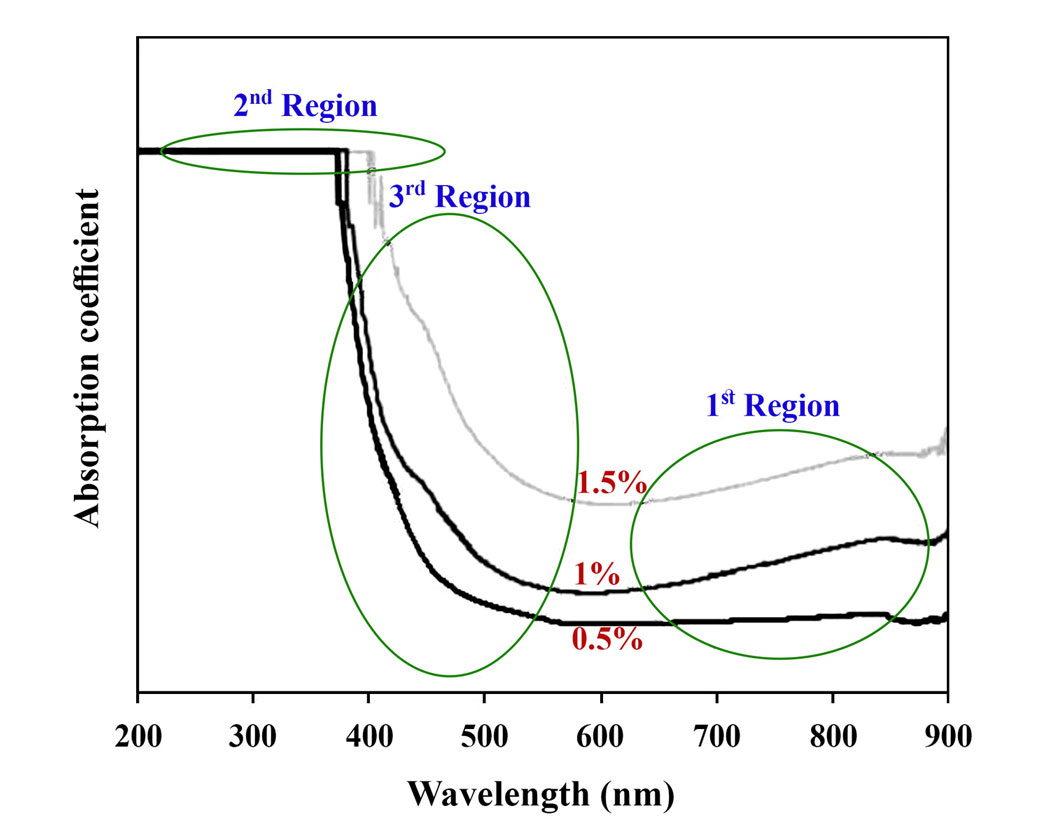
In this study, the structural chemistry of Fe3+ doped Li2O–Al2O3–SiO2–TiO2 (LAST) glasses has been analyzed utilizing UV-Vis spectroscopy. Optical parameters like absorption and extinction coefficients, indirect and direct optical band gaps, Urbach energy as well as Fermi energy level of samples were estimated via their absorption spectra. Then, it was tried to make a relationship between the variation of mentioned parameters and structural chemistry of different doped samples. Results of the investigation illustrated that even a little change in the microstructure of glassy samples has an effect on optical parameters and accordingly it could be sensible. Furthermore, it was revealed that Fe3+ ions have the role of network forming in the structure of glass by increasing the formation of bridging oxygens (BOs) in the matrix.
Downloads
References
Copyright (c) 2022 Aida Faeghinia

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












