Thermodynamically study of phase formation of Ni-Ti-Si nanocomposites produced by self-propagating high-temperature synthesis method
- 1 School of Metallurgy & Materials Engineering, Iran University of Science and Technology, Narmak, Tehran, Iran
- 2 Nanotechnology Research & Application Center, Sabanci University, Tuzla 34956, Istanbul, Turkey
- 3 Materials Engineering Department, University of Tabriz, Tabriz, Iran
- 4 Faculty of Mechanical Engineering, University of Tabriz, Tabriz, Iran
- 5 Department of Physical Electronics, Masaryk University, Brno, Czech
Abstract
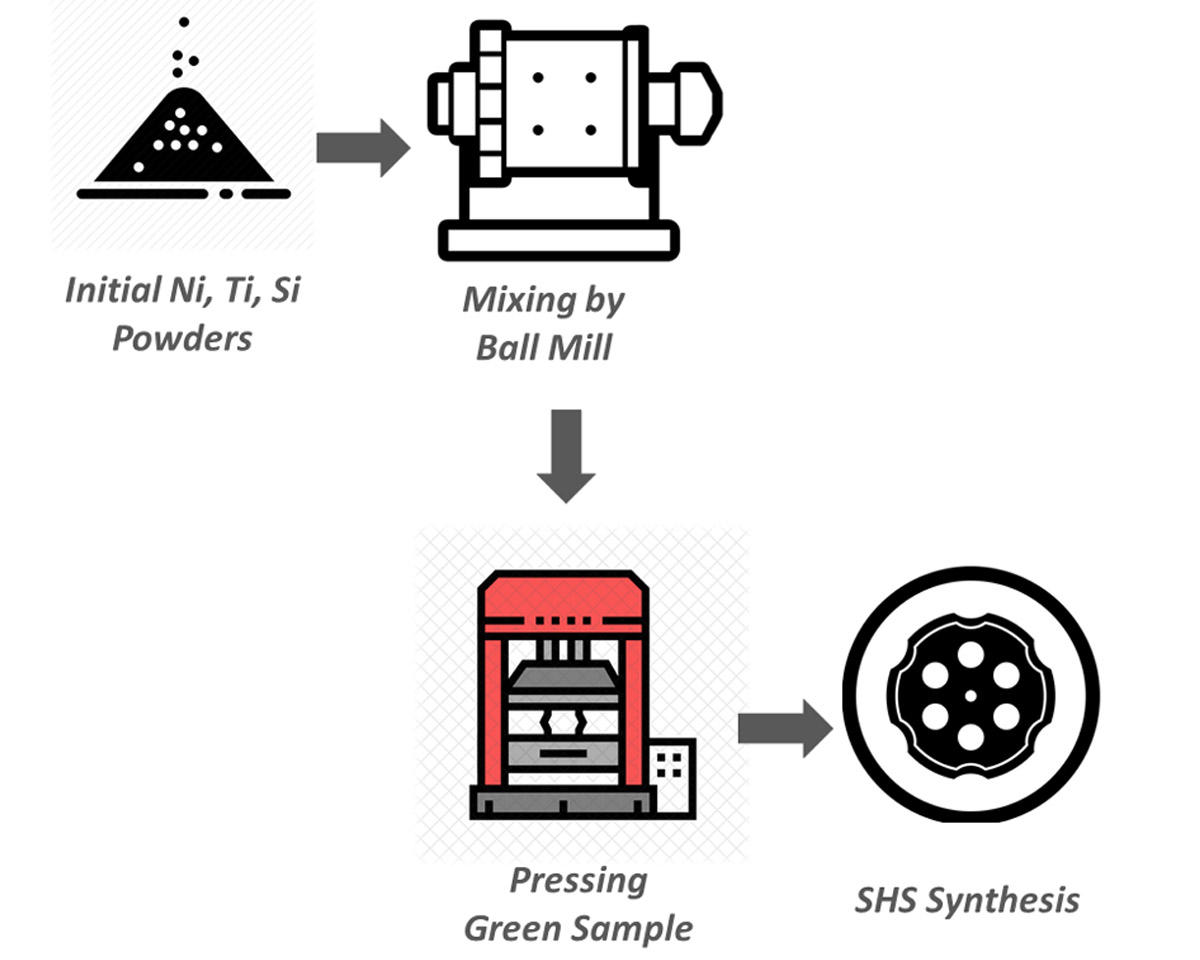
Understanding the phase formation mechanisms in self-propagating high-temperature synthesis from the thermodynamical aspect of view is important. In this study, the phase formation of the ternary system of nickel-titanium-silicon was studied by using the HSC software V6.0, and phase formation is predicted by calculating the adiabatic temperature of exothermic reaction between reagents. Then, by using X-ray diffractometer analysis, the results of the simulation were evaluated by experimental achievements. Results showed a good correlation between thermodynamical calculation and prediction with experimental. It could be concluded that the equilibrium mechanism is the dominant mechanism in phase formation in the SHS synthesis method. NiTiSi solid solution phase is obtained from the reaction between Ti5Si3 and Ni2Si and Ni.
Downloads
References
Copyright (c) 2021 Hossein Aghajani, Arvin Taghizadeh Tabrizi, Salva Arabpour Javadi, Mohammad Ehsan Taghizadeh Tabrizi, Aytak Homayouni, Sahand Behrangi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












