Synthesis and characterization of ZnS and Ag-ZnS nanoparticles for photocatalytic degradation of aqueous pollutants
- 1 Faculty of Materials and Metallurgical Engineering, Semnan University, Semnan, Iran
- 2 Department of Nanotechnology, Faculty of New Sciences and Technologies, Semnan University, Semnan, Iran
Abstract
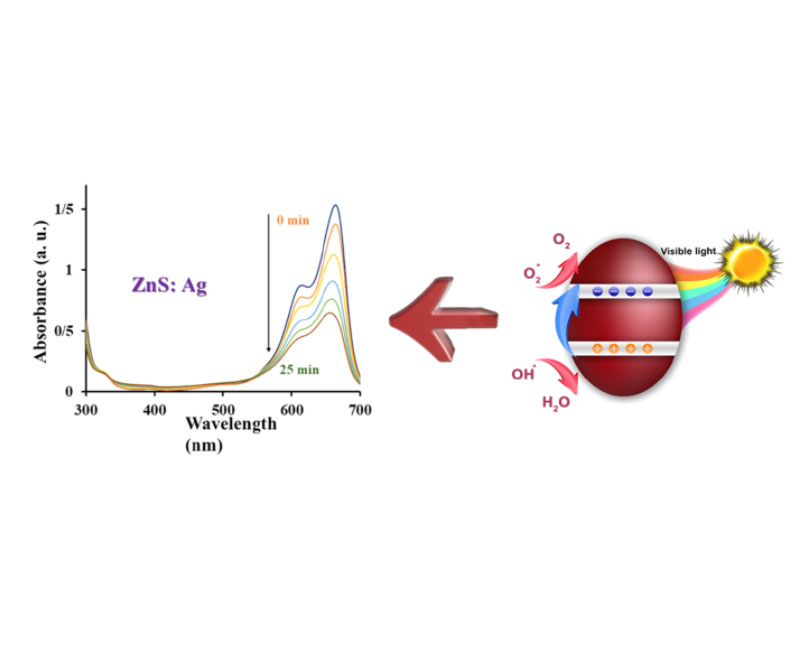
Photocatalytic degradation has drawn much interest recently as a substitute technique for eliminating environmental contaminants from the aqueous phase. In this study, pure and Ag-doped zinc sulfide (ZnS) nanoparticles were synthesized for the photocatalytic degradation of methylene blue (MB) under UVA light irradiation using a simple chemical co-precipitation method. The nanopowders' structural, optical, morphological, and chemical properties were characterized using XRD, FTIR, UV-Vis, and FESEM techniques. XRD analysis confirmed the hexagonal crystal structure of the nanoparticles, while FTIR identified stretching vibrations corresponding to O–H, C–H, C=O, C–N, and Zn–S bonds. The UV-Vis analysis revealed an optical band gap in the range of 5.2–5.4 eV. Photocatalytic performance tests under UVA light demonstrated that Ag doping significantly enhanced the photocatalytic efficiency of ZnS nanoparticles in degrading MB. Upon exposure to UVA light, the synthesized Ag-ZnS nanoparticles achieved impressive decolorization efficiency within 25 minutes, compared to 35 minutes for pure ZnS. The findings indicate that Ag-ZnS is a highly promising photocatalyst for the efficient removal of aqueous pollutants, including methylene blue dye.
Downloads
References
Copyright (c) 2024 Amir Hossein Afzali, Arshia Seddiqi, Zahra Akbari, Maryam Hajiebrahimi, Sanaz Alamdari, Omid Mirzaee

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












