Synthesis and characterization of mullite (3Al2O3.2SiO2) sol by sol-gel route using inorganic salts
- 1 Department of Materials Science and Engineering, University of Tabriz, Tabriz, Iran
Abstract
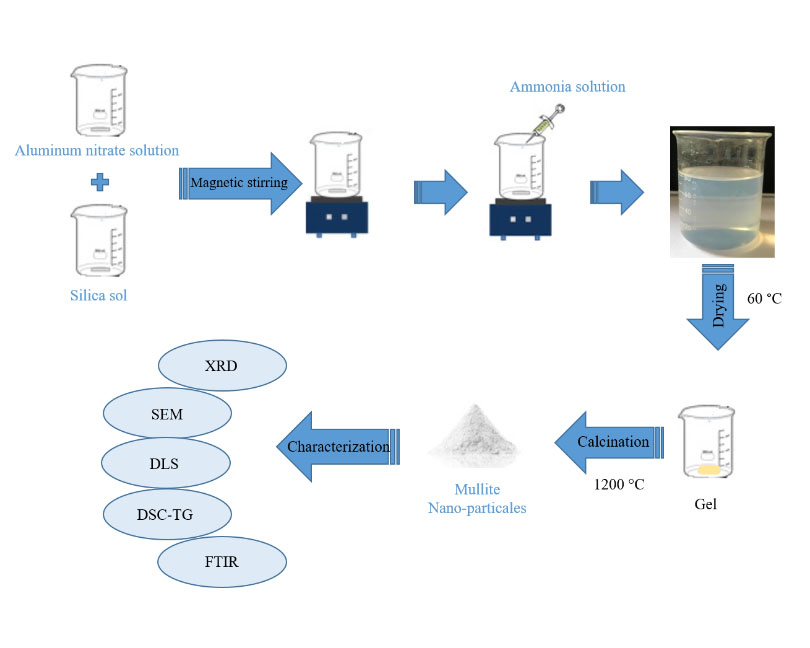
Mullite, also referred to as 3Al2O3.2SiO2, is recognized as the only chemically stable intermediate phase in the SiO2–Al2O3 system, as indicated by mineralogical studies. Several synthesis techniques can be employed to obtain mullite. In this research, the synthesis of mullite particles is aimed to be achieved via the sol-gel route using inexpensive materials, specifically silica sol and aluminum nitrate hydrate [(Al(NO3)3.9H2O] as sources for silica and alumina, respectively. The article is organized into two sections which describe and discuss the systematic synthesis of the mullite sol. The first section emphasizes the influence of the stoichiometric values of Al and Si elements on the formation of the mullite phase at 1200 °C. The effects of sintering temperature on the microstructure and composition of the synthesized mullite sol, with a 3:1 alumina-to-silica ratio, are discussed in the following section. This includes pH, density, solid content, particle size distribution, thermal analysis, phase evolution with temperature, nature of bonds, and microstructural analysis. The XRD results for the mullite sol with a 3:1 alumina-to-silica ratio show strong crystalline diffraction peaks of the mullite phase and the absence of a free silica phase at 1200 °C. The solution exhibits a clear, stable, and homogeneous appearance, with a density of 1.17 g/cm3, a pH ranging from 4 to 5, and a solid content of approximately 15%, measured after heating at 1000 °C for 2 h.
Downloads
References
Copyright (c) 2024 Sahar Sajjadi Milani, Mahdi Ghassemi Kakroudi, Nasser Pourmohammadi Vafa

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












