Evaluation of the contribution of media derived from various animal livers on the production of Lucilia sericata
- 1 Department of Medical Microbiology, İstanbul University-Cerrahpaşa School of Medicine, İstanbul, Turkey
- 2 Department of Biophysics, İstanbul University-Cerrahpaşa School of Medicine, İstanbul, Turkey
- 3 Department of Medical Biology, İstanbul University-Cerrahpaşa School of Medicine, İstanbul, Turkey
- 4 Institute of Forensic Sciences and Legal Medicine, Istanbul University-Cerrahpasa, Istanbul, Turkey
- 5 Dogus University, School of Vocational, Department of Pathology Laboratory Techniques, Istanbul, Turkey
- 6 Department of Medical Laboratory Techniques, Şabanözü Vocational School, Çankırı Karatekin University, Çankırı, Turkey
Abstract
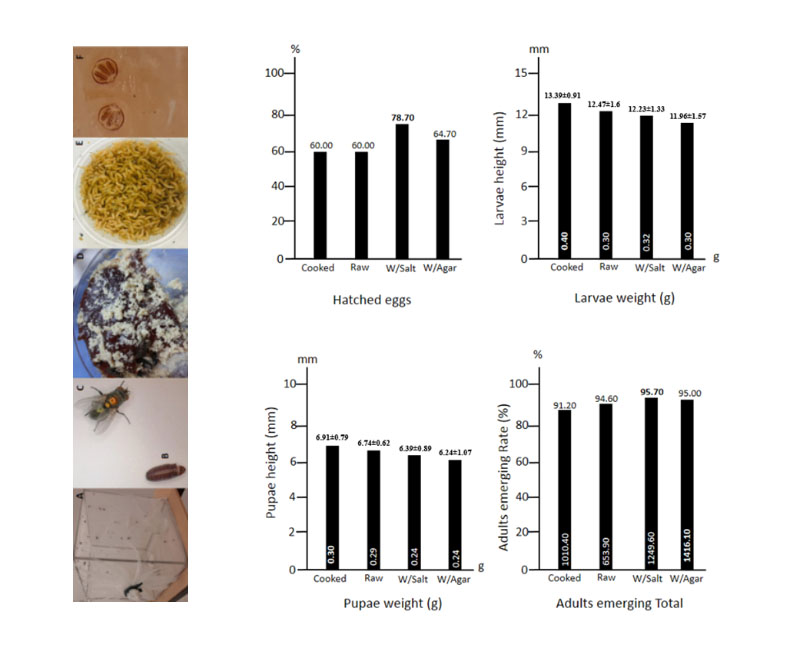
The effects of liver from different animals and agar- media on the production of Lucilia sericata (Meigen 1826) larvae were investigated to determine the best medium for producing larvae for wound therapy. The research was conducted in two phases. The best liver for generating L. sericata larvae was determined in the first phase, using media with beef, porcine, lamb, and chicken livers gelled with agar. In the first phase of the research, it was established that chicken liver was acceptable since the number of flies emerging from puparia was the highest at 80.75%. The preparation and content of the best medium for developing L. sericata larvae were determined in the second phase using chicken liver, raw, cooked, agar, and agar + salt. The number of flies emerging from puparia on the medium with chicken liver + salt + agar was 95.7% in the second phase, followed by 95% of flies coming out of the pupa in the medium prepared with chicken liver and agar. Finally, as the number of flies developing in these two mediums was not significantly different, we believe that the chicken liver and agar medium are most suitable for developing larvae.
Downloads
References
Copyright (c) 2024 Erdal Polat, Emre Deymenci, Kübra Tugtekin, Merve Cil, Zahra Bahararjmand, Serhat Sirekbasan

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












