Unlocking the potential of aromatase inhibitors: recent advances in drug design, synthesis, docking activity, and in vitro bioactivity evaluations
- 1 Faculty of Pharmacy, Cyprus International University, Nicosia 99258, Northern Cyprus via Mersin 10, Turkey
- 2 Faculty of Pharmacy, Eastern Mediterranean University, Famagusta 99628, Northern Cyprus via Mersin 10, Turkey
Abstract
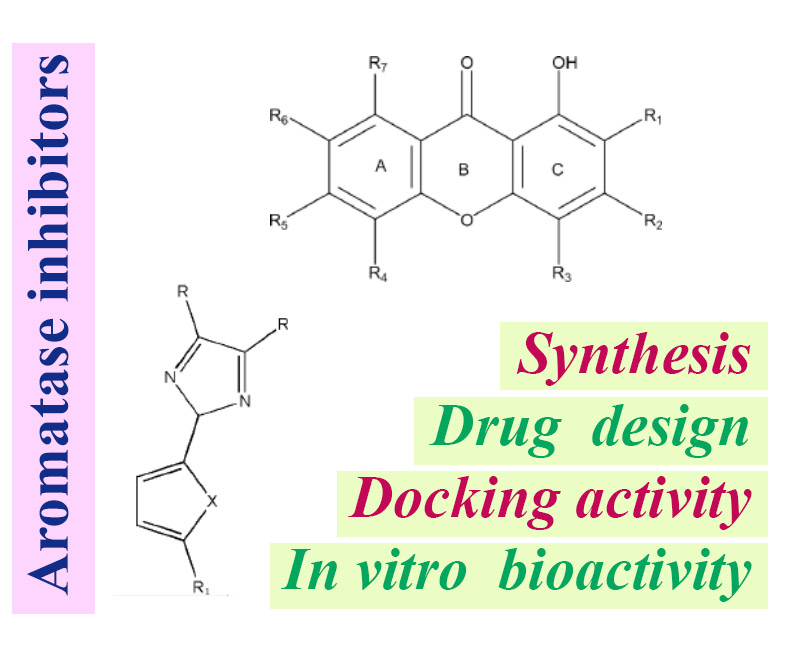
Breast cancer, a global health concern claiming approximately 685,000 lives in 2020, necessitates continual advancements in therapeutic strategies. Estrogen and aromatase play pivotal roles in hormone-responsive breast cancer, with 80% of patients exhibiting estrogen receptor-positive tumors. Aromatase inhibitors (AIs), notably non-steroidal inhibitors like anastrozole and letrozole, have significantly improved outcomes, yet challenges persist, including side effects. This review focuses on recent developments in AIs, exploring xanthone derivatives, imidazole derivatives, and curcumin derivatives as potential inhibitors of aromatase. Molecular docking studies, employing Auto Dock and other tools, reveal the binding affinities and interactions of these compounds with the aromatase enzyme. Among xanthones, Erythrommone emerges as a potent inhibitor, holding promise for clinical trials. Imidazole derivatives, synthesized through the Debus-Radziszewski reaction, demonstrate anticancer potential, with compounds like 1a exhibiting superior efficacy against MCF7 cells. ADME-Tox analyses indicate promising drug-likeness but reveal potential mutagenic effects and environmental impacts. Curcumin derivatives, particularly 1,5-diaryl-1,4-pentadien-3-ones, present alternatives to address curcumin's bioavailability challenges. A study of 25 compounds (DKC) identifies DKC-10 as a potent inhibitor, outperforming established breast cancer drugs in terms of binding affinity and interactions with aromatase and ERα+ receptors. These findings underscore the importance of exploring diverse chemical structures in developing AIs, paving the way for more effective and well-tolerated therapeutics. The integration of computational techniques, such as molecular docking studies, accelerates drug discovery by predicting interactions at the molecular level. Overall, this comprehensive review provides valuable insights into the evolving landscape of aromatase inhibitors, offering a roadmap for future research and the development of advanced breast cancer therapeutics.
Downloads
References
Copyright (c) 2023 Niloufar Moharrer Navaei, Narvan Moharrer Navaei

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.












