Molecular hydrogen production by radiolysis of water on the surface of nano-ZrO2 under the influence of gamma rays
- 1 Institute of Radiation Problems, Azerbaijan National Academy of Sciences, AZ 1143-Baku, Azerbaijan
Abstract
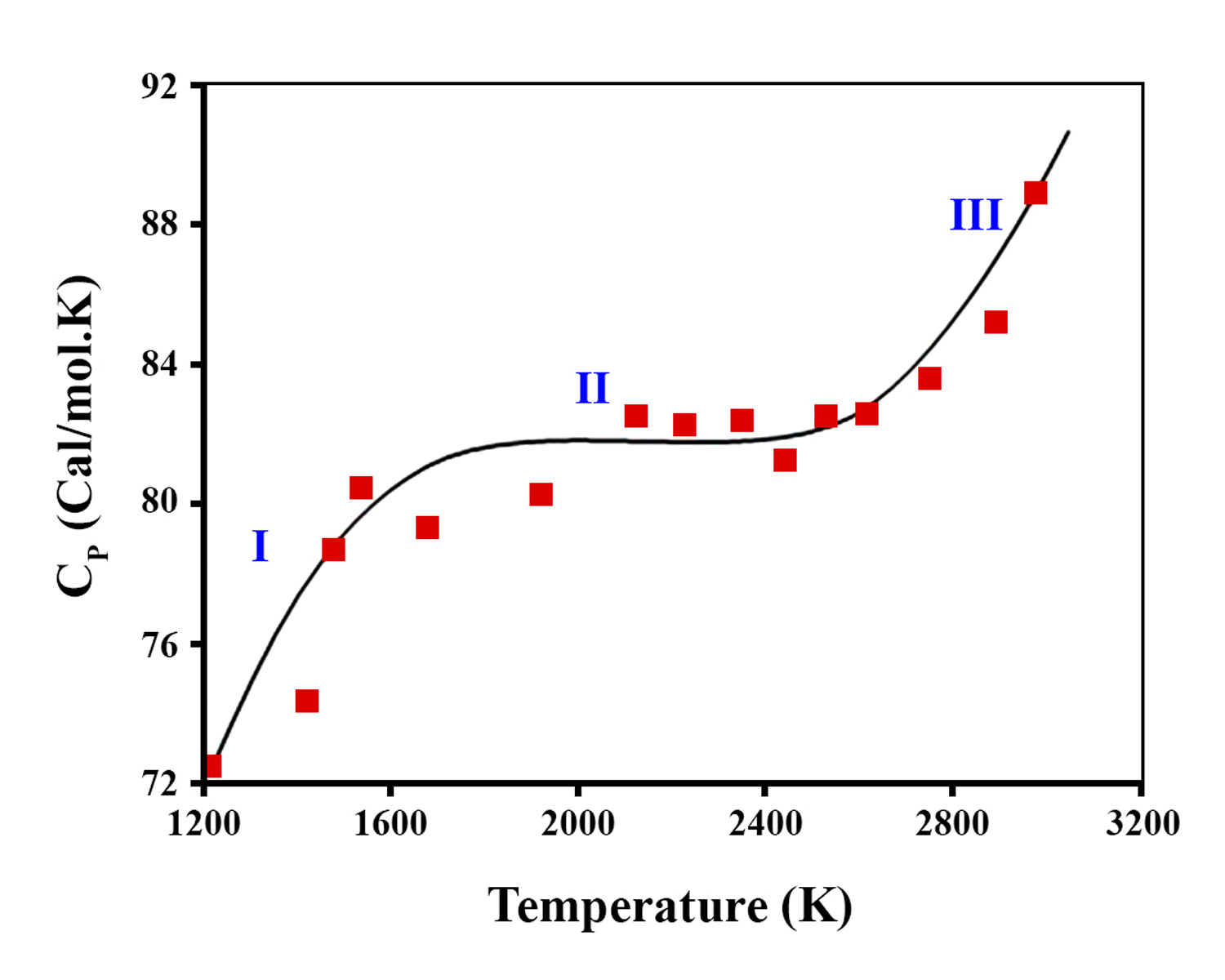
In this research, the radiation-heterogeneous processes of water decomposition on the surface of zirconium dioxide nanoparticles (n-ZrO2) were studied. The kinetics of buildup of molecular hydrogen during the radiolytic processes of water decomposition was also examined. The production of H2 and H2O2 through water radiolysis was investigated to develop a computational model and disclose the kinetic behavior of water radiolysis. The enthalpy of ZrO2 nanoparticles was studied at the temperature range T=1200-2900 K, in which ZrO2 nanoparticles has a two-phase transition. Some of the electrons were transported to the surface of the nanoparticles during the physical and physicochemical stages of the process and emitted into the water. At the same time, the migration of energy carriers in radioactively active oxide compounds changed at different intervals depending on the composition, structural stability, and electro-physical properties of the oxides.
Downloads
References
Copyright (c) 2022 Gunel Imanova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.